Biological Burden - Bioburden Analysis
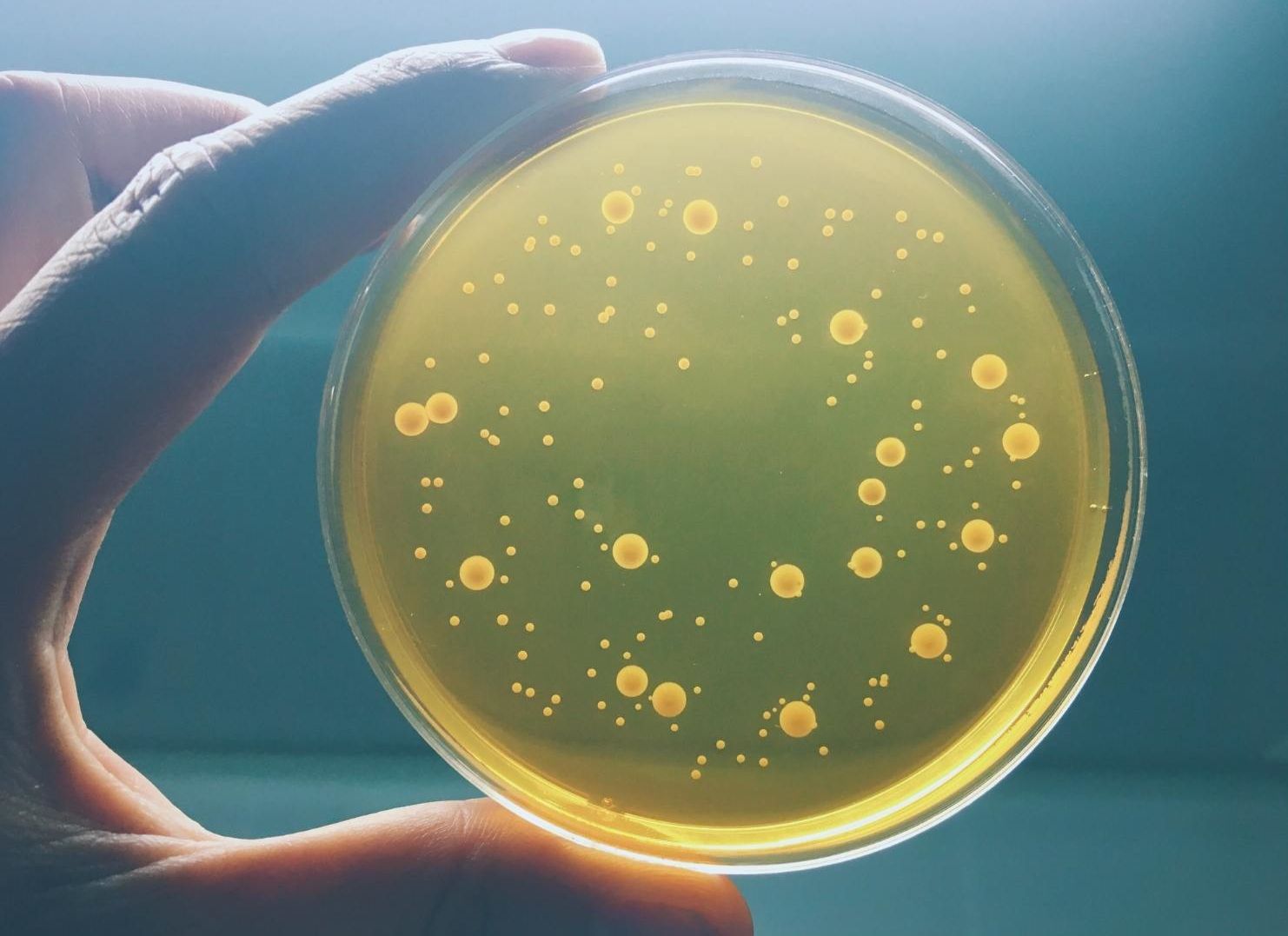
Bioburden Analysis is a microbiological test conducted after the production process and before the sterilization of medical devices. This test is performed to calculate the total biological load that occurs during the production of medical devices and to determine the number of viable microorganisms.
This analysis guides manufacturers in evaluating and optimizing the effectiveness of sterilization processes. Conducted before the sterilization of medical devices, this test ensures the safety of the devices and their compliance with hygiene standards.
At Oxigen Industrial Laboratory, we perform Bioburden Tests in accordance with ISO 11737-1 standards under accreditation. This analysis, which is of critical importance in the healthcare sector, helps ensure the quality and safety compliance of your products.
